Ocean acidification dramatically alters marine ecosystems by reducing the pH levels of seawater due to increased carbon dioxide absorption. This process threatens coral reefs, shellfish, and biodiversity, disrupting food chains and coastal economies. Understanding its impacts is crucial for developing strategies to mitigate damage and protect ocean health.
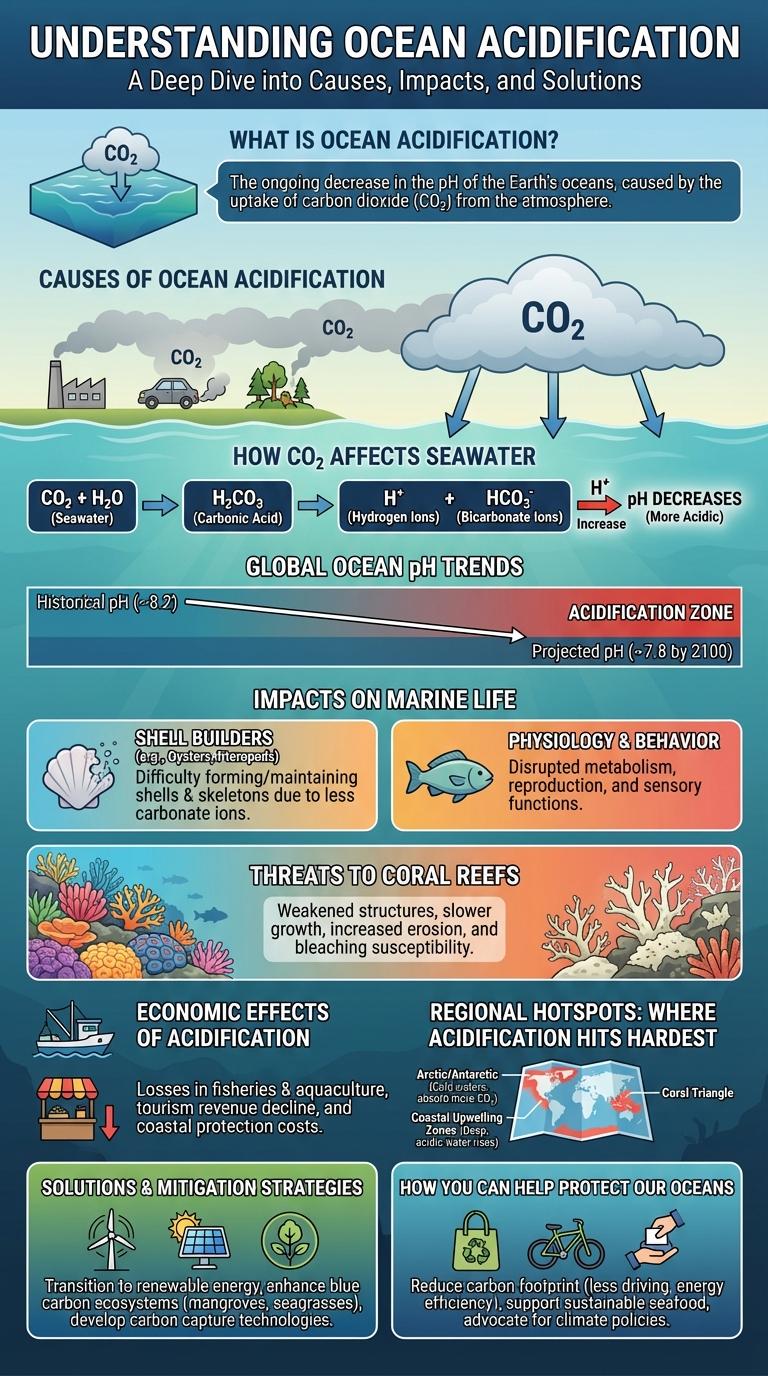
What Is Ocean Acidification?
Ocean acidification refers to the ongoing decrease in the pH levels of the Earth's oceans caused by the absorption of excess atmospheric carbon dioxide (CO2). When CO2 dissolves in seawater, it forms carbonic acid, leading to increased acidity that disrupts marine ecosystems. This process poses significant threats to coral reefs, shellfish, and other marine organisms that rely on calcium carbonate for their skeletal structures.
Causes of Ocean Acidification
What are the primary causes of ocean acidification?
Ocean acidification occurs mainly due to the increased absorption of carbon dioxide (CO2) from the atmosphere by seawater. Human activities such as burning fossil fuels and deforestation release large amounts of CO2, driving this chemical change in the oceans.
How CO₂ Affects Seawater
Ocean acidification occurs when seawater absorbs carbon dioxide (CO2) from the atmosphere. This process lowers the pH level of the ocean, making it more acidic.
When CO2 dissolves in seawater, it reacts to form carbonic acid. Carbonic acid then dissociates into hydrogen ions and bicarbonate, increasing acidity. Higher acidity reduces carbonate ions, which are essential for marine organisms like corals and shellfish to build their skeletons and shells.
Global Ocean pH Trends
| Year | Average Ocean pH |
|---|---|
| 1750 (Pre-Industrial) | 8.2 |
| 1950 | 8.1 |
| 2000 | 8.05 |
| 2020 | 8.01 |
| Projected 2050 | 7.9 |
Global ocean pH has declined steadily since pre-industrial times due to increased atmospheric carbon dioxide absorption. This acidification impacts marine ecosystems by reducing carbonate ion availability, essential for shell-building organisms.
Impacts on Marine Life
Ocean acidification significantly alters marine ecosystems by changing water chemistry, affecting species survival and biodiversity. These chemical changes reduce the availability of calcium carbonate, essential for shell-forming marine organisms.
- Coral Reef Degradation - Acidic waters weaken coral skeletons, leading to fragile reefs and loss of habitat for marine species.
- Shellfish Vulnerability - Lower pH levels hinder shell formation in mollusks, decreasing their growth and survival rates.
- Disrupted Food Chains - Changes in plankton populations affect the entire marine food web, impacting fish and marine mammals.
Threats to Coral Reefs
Ocean acidification poses a significant threat to coral reef ecosystems by disrupting their delicate chemical balance. Increased CO2 absorption lowers pH levels, impairing coral growth and resilience.
Coral reefs support biodiversity and protect coastlines, but acidification weakens their calcium carbonate structures, making them more susceptible to damage.
- Reduced Calcification Rates - Acidified waters decrease the availability of carbonate ions essential for coral skeleton formation.
- Increased Coral Bleaching - Lower pH levels stress corals, leading to higher instances of bleaching and mortality.
- Weakened Reef Structures - Erosion accelerates as coral skeletons become brittle, compromising reef habitats.
Economic Effects of Acidification
Ocean acidification significantly impacts marine industries by reducing fish stocks and shellfish productivity. Economic losses in fisheries and aquaculture could reach billions annually due to decreased species growth and survival rates.
Tourism also suffers as coral reefs degrade, diminishing the natural attractions that support local economies. Coastal communities face increased financial strain from the combined effects on seafood supply and tourism revenue.
Regional Hotspots: Where Acidification Hits Hardest
Ocean acidification intensifies in specific regional hotspots where CO2 absorption significantly lowers seawater pH levels. These areas face severe impacts on marine ecosystems and biodiversity due to heightened acidic conditions.
- Arctic Ocean - Rapid ice melt and cold water increase CO2 solubility, causing accelerated acidification in this fragile environment.
- California Current - Upwelling of CO2-rich deep waters produces low pH zones, threatening shellfish and other marine species.
- Coral Triangle - High biodiversity combined with acidification stresses coral reefs, impairing growth and structural integrity.
Monitoring these regional hotspots is essential for targeted conservation and mitigation strategies against ocean acidification.
Solutions & Mitigation Strategies
Ocean acidification poses a severe threat to marine ecosystems by lowering pH levels in seawater, impacting coral reefs, shellfish, and biodiversity. Effective solutions focus on reducing carbon dioxide emissions, the primary cause of acidification, through innovative energy technologies and sustainable practices.
Mitigation strategies include enhancing marine protected areas to support ecosystem resilience and investing in research for acidification-resistant marine species. Restoration of coastal habitats like mangroves and seagrasses also plays a crucial role by absorbing CO2 and buffering local pH changes.
