Carbon compounds form the backbone of organic chemistry, encompassing a vast array of molecules essential to life and industrial applications. These compounds vary from simple hydrocarbons to complex biomolecules, each with unique structures and functions. Understanding their properties and classifications provides insight into chemical reactions and environmental impact.
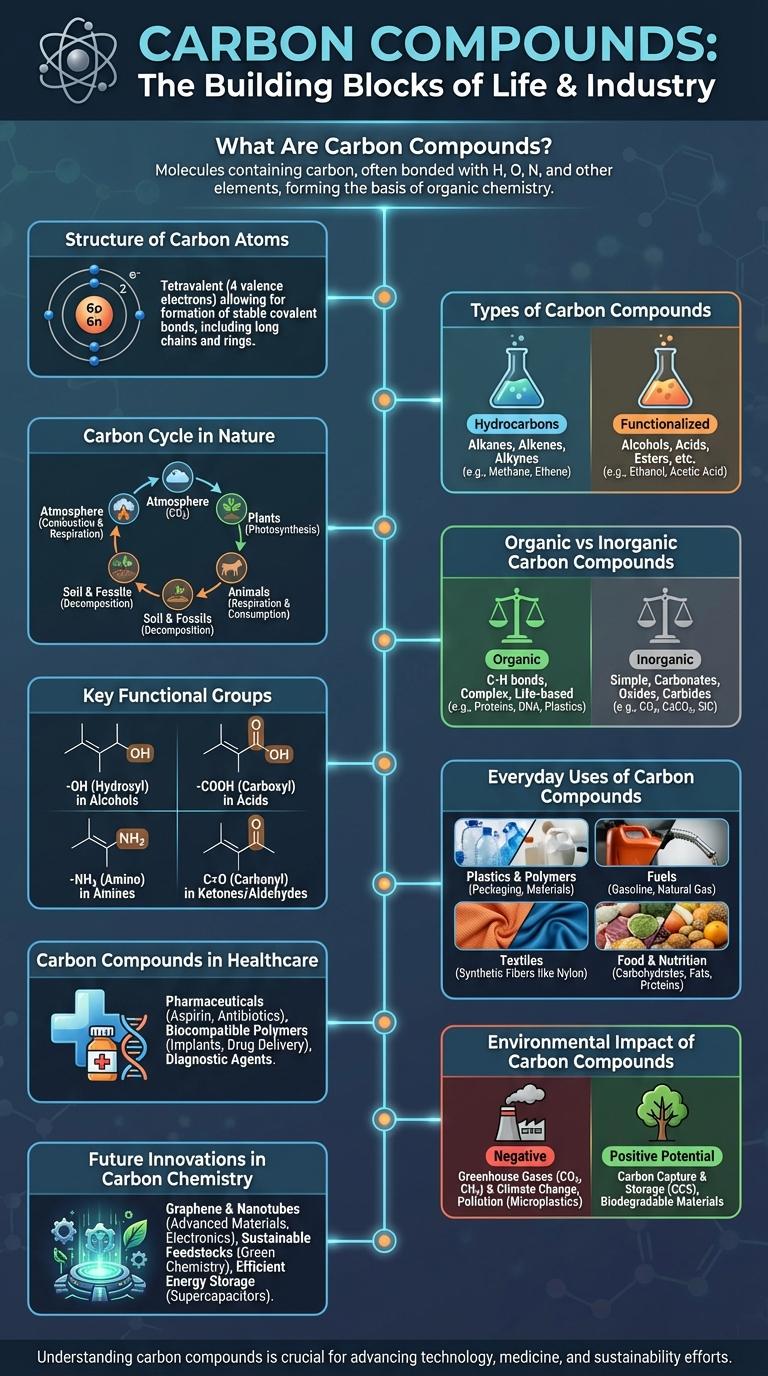
What Are Carbon Compounds?
Carbon compounds are chemical substances consisting primarily of carbon atoms bonded with other elements such as hydrogen, oxygen, and nitrogen. These compounds form the basis of organic chemistry and include a vast variety of molecules essential for life. Examples of carbon compounds include carbohydrates, proteins, lipids, and nucleic acids.
Structure of Carbon Atoms
| Characteristic | Details |
|---|---|
| Atomic Number | 6 |
| Electron Configuration | 1s2 2s2 2p2 |
| Valence Electrons | 4 |
| Bonding Capability | Forms up to 4 covalent bonds |
| Hybridization Types | sp3, sp2, sp |
Types of Carbon Compounds
Carbon compounds form the basis of organic chemistry and are essential to life on Earth. These compounds vary widely in structure and function, ranging from simple molecules to complex macromolecules.
- Hydrocarbons - Composed solely of carbon and hydrogen atoms, hydrocarbons include alkanes, alkenes, and alkynes, serving as fuels and raw materials in industry.
- Alcohols - Contain one or more hydroxyl (-OH) groups attached to carbon atoms, playing key roles in solvents, antiseptics, and biochemical processes.
- Carboxylic Acids - Characterized by the presence of a carboxyl group (-COOH), these compounds are fundamental in metabolism and the manufacturing of polymers and pharmaceuticals.
Carbon Cycle in Nature
The carbon cycle is a complex process that moves carbon through Earth's atmosphere, biosphere, hydrosphere, and geosphere. It plays a crucial role in regulating global climate and sustaining life.
- Photosynthesis - Plants absorb carbon dioxide from the atmosphere and convert it into organic compounds.
- Respiration - Organisms release carbon dioxide back into the atmosphere by breaking down organic molecules.
- Decomposition - Microorganisms break down dead matter, returning carbon to the soil and atmosphere.
Carbon storage in oceans, soils, and fossil fuels regulates atmospheric carbon levels and influences climate change.
Organic vs Inorganic Carbon Compounds
Carbon compounds are categorized into organic and inorganic groups based on their chemical structure and bonding. Understanding the differences is essential for chemistry, biology, and environmental science.
- Organic Carbon Compounds - Contain carbon atoms bonded primarily to hydrogen, oxygen, and nitrogen, forming complex molecules like carbohydrates and proteins.
- Inorganic Carbon Compounds - Include carbonates, oxides, and cyanides, typically lacking carbon-hydrogen bonds and found in minerals and gases.
- Structural Complexity - Organic compounds exhibit diverse structures such as chains, rings, and branches, while inorganic compounds have simpler molecular forms.
Key Functional Groups
Carbon compounds form the backbone of organic chemistry, with various functional groups determining their chemical behavior. Key functional groups include hydroxyl, carbonyl, carboxyl, amino, and phosphate groups.
Hydroxyl groups (-OH) are found in alcohols and influence polarity and solubility. Carbonyl groups (C=O) appear in aldehydes and ketones, crucial for reactivity in many biological molecules.
Everyday Uses of Carbon Compounds
Carbon compounds are essential in daily life, forming the basis of many materials and products we use. Common examples include plastics, fuels, medicines, and foods, all relying on carbon's versatile bonding properties. Understanding carbon compounds helps highlight their impact on technology, health, and the environment.
| Carbon Compound | Everyday Use |
|---|---|
| Hydrocarbons | Fuel for vehicles and heating |
| Polymers | Plastics for packaging and containers |
| Carbohydrates | Energy source in food |
| Pharmaceuticals | Medicines and vaccines |
| Carbonates | Building materials like cement |
Carbon Compounds in Healthcare
How do carbon compounds impact healthcare innovations? Carbon compounds form the backbone of many pharmaceuticals, enabling the development of effective medicines. Their versatile chemical properties allow for targeted treatment of diseases with minimal side effects.
Environmental Impact of Carbon Compounds
Carbon compounds play a significant role in environmental processes and pollution. They include a wide range of substances such as carbon dioxide, methane, and organic pollutants.
Carbon dioxide contributes to global warming by trapping heat in the atmosphere. Methane is a potent greenhouse gas with a much higher heat-trapping ability than CO2 over a short period.
