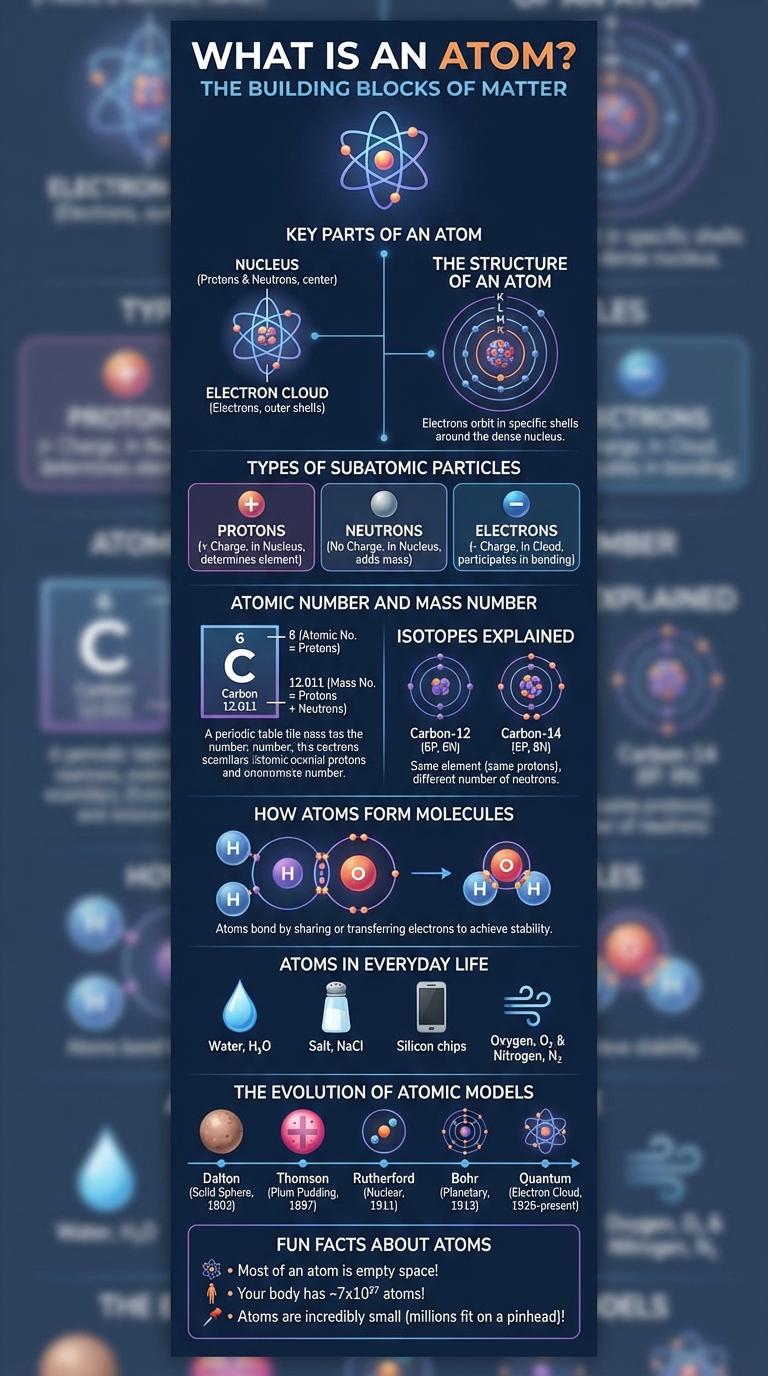
Atoms are the fundamental building blocks of matter, comprising protons, neutrons, and electrons arranged in a dense nucleus surrounded by an electron cloud. Understanding atomic structure reveals how elements interact and combine to form everything in the universe. This infographic visually breaks down the components, properties, and significance of atoms in science and daily life.
What is an Atom?
An atom is the smallest unit of ordinary matter that forms the building blocks of all substances. It consists of a nucleus containing protons and neutrons, surrounded by electrons in defined orbitals.
Atoms combine to create molecules, which make up everything we see around us. The number of protons defines the element, while the arrangement of electrons influences chemical properties. Understanding atoms helps explain the structure and behavior of matter at a fundamental level.
Key Parts of an Atom
| Part of Atom | Description |
|---|---|
| Proton | Positively charged particle found in the nucleus; determines atomic number. |
| Neutron | Neutral particle in the nucleus; contributes to atomic mass and isotope variation. |
| Electron | Negatively charged particle orbiting the nucleus in energy levels or shells. |
| Nucleus | Central core composed of protons and neutrons; holds most of the atom's mass. |
| Electron Cloud | Region around nucleus where electrons are likely to be found; defines atom's size. |
The Structure of an Atom
What is the structure of an atom?
An atom consists of a nucleus at its center, containing protons and neutrons. Electrons orbit the nucleus in energy levels or shells, creating the overall structure of the atom.
Types of Subatomic Particles
Atoms are the fundamental building blocks of matter, composed of smaller particles called subatomic particles. These particles determine the atom's properties and behavior.
- Protons - Positively charged particles found in the nucleus that define the atomic number.
- Neutrons - Neutral particles located in the nucleus that add mass and influence stability.
- Electrons - Negatively charged particles orbiting the nucleus that participate in chemical bonding.
Understanding subatomic particles is essential for grasping atomic structure and chemical interactions.
Atomic Number and Mass Number
The atomic number represents the number of protons in an atom's nucleus, determining the element's identity. Each element has a unique atomic number, which is essential for organizing the periodic table.
The mass number is the total count of protons and neutrons in the nucleus, indicating the atom's overall mass. Variations in mass number result in different isotopes of the same element.
Isotopes Explained
Isotopes are variants of a chemical element that share the same number of protons but differ in neutron count. These differences affect atomic mass but not chemical properties.
Understanding isotopes is essential in fields like medicine, archaeology, and nuclear energy.
- Definition of Isotopes - Atoms of the same element with different numbers of neutrons.
- Atomic Mass Variation - Isotopes have different atomic masses due to neutron differences.
- Applications of Isotopes - Used in radiocarbon dating, medical imaging, and nuclear reactors.
How Atoms Form Molecules
Atoms are the basic units of matter that bond together to form molecules. The way atoms share or transfer electrons determines the types of molecules they create.
- Electron Sharing - Atoms form covalent bonds by sharing pairs of electrons to achieve stability.
- Electron Transfer - Ionic bonds result from atoms transferring electrons, creating charged ions that attract each other.
- Bond Strength - The strength and type of bonds influence the structure and properties of molecules.
Atoms in Everyday Life
Atoms are the fundamental building blocks of all matter, making up everything we see and touch every day. From the air we breathe to the food we eat and the technology we use, atoms form the basis of all physical substances. Understanding atoms helps us unlock innovations in medicine, energy, and materials science that impact daily life.
The Evolution of Atomic Models
The evolution of atomic models reflects the progressive understanding of atomic structure from ancient philosophical ideas to modern quantum mechanics. Early models began with Dalton's solid sphere concept, followed by Thomson's plum pudding model, Rutherford's nuclear model, and Bohr's planetary model. The latest advancements incorporate quantum mechanics, describing electrons as probabilistic clouds rather than fixed orbits.
