Chemistry infographics visually simplify complex scientific concepts, making information more accessible and engaging. They highlight key elements such as molecular structures, chemical reactions, and periodic table trends through clear graphics and concise data. This approach enhances understanding and retention for students, educators, and enthusiasts alike.
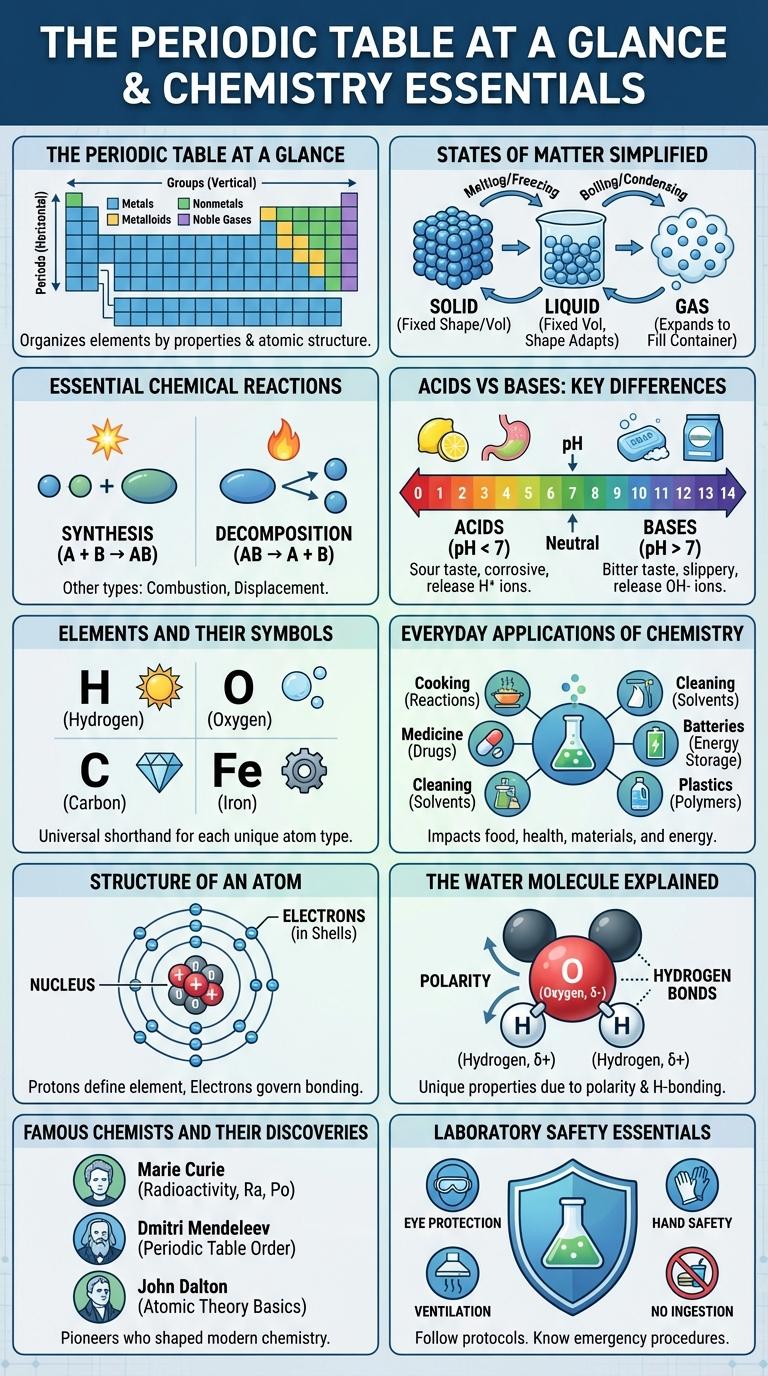
The Periodic Table at a Glance
The periodic table organizes all known chemical elements based on their atomic number, electron configurations, and recurring chemical properties. It provides a systematic framework to understand element behavior and relationships.
Elements are arranged in rows called periods and columns known as groups or families, each sharing similar characteristics. This arrangement helps predict element reactions and properties, making it essential in chemistry.
States of Matter Simplified
| State of Matter | Key Characteristics |
|---|---|
| Solid | Definite shape and volume; particles tightly packed in fixed positions; low compressibility; strong intermolecular forces. |
| Liquid | Definite volume but no fixed shape; particles close but can slide past each other; moderate compressibility; moderate intermolecular forces. |
| Gas | No definite shape or volume; particles far apart and move freely; high compressibility; weak intermolecular forces. |
| Plasma | Ionized gas with free electrons; conducts electricity; extremely high energy state; found in stars and fluorescent lights. |
Essential Chemical Reactions
Chemistry revolves around understanding essential chemical reactions that drive natural and industrial processes. These reactions involve the transformation of substances through breaking and forming chemical bonds.
Key chemical reactions include synthesis, decomposition, single replacement, double replacement, and combustion. Synthesis reactions combine elements or compounds to form a more complex product. Combustion reactions release energy by reacting a substance with oxygen, widely used in energy production.
Acids vs Bases: Key Differences
Acids and bases are fundamental chemical substances with distinct properties and behaviors. Understanding their differences is essential in chemistry applications and reactions.
Acids release hydrogen ions (H+) in aqueous solutions, while bases release hydroxide ions (OH-).
- pH Levels - Acids have a pH less than 7, indicating higher acidity; bases have a pH greater than 7, indicating alkalinity.
- Taste and Texture - Acids typically taste sour and can corrode metals; bases taste bitter and feel slippery or soapy.
- Reactivity with Indicators - Acids turn blue litmus paper red, whereas bases turn red litmus paper blue.
Elements and Their Symbols
Chemistry studies the properties and interactions of elements, each represented by unique symbols. These symbols are internationally standardized for consistency in scientific communication.
- Hydrogen (H) - The lightest element and most abundant in the universe, essential for water formation.
- Oxygen (O) - Vital for respiration, it supports combustion and constitutes about 21% of Earth's atmosphere.
- Carbon (C) - The foundation of organic chemistry, it forms the backbone of all known life forms.
- Iron (Fe) - A key metal element used in construction and manufacturing, known for its magnetic properties.
- Gold (Au) - A precious metal valued for its resistance to corrosion and electrical conductivity.
Everyday Applications of Chemistry
Chemistry plays a vital role in everyday life, from the food we eat to the cleaning products we use. It helps in the development of medicines, fuels, and materials that improve our quality of life. Understanding chemical reactions enables innovations in cosmetics, textiles, and environmental solutions.
Structure of an Atom
The structure of an atom consists of a central nucleus containing protons and neutrons, surrounded by electrons in orbitals. Protons carry a positive charge, neutrons are neutral, and electrons have a negative charge, maintaining overall atomic stability. Atomic behavior and chemical properties are determined primarily by the number and arrangement of electrons.
The Water Molecule Explained
What makes the water molecule essential for life? Water is composed of two hydrogen atoms and one oxygen atom, creating a polar molecule. This polarity allows water to dissolve many substances, supporting vital biological processes.
| Property | Description |
|---|---|
| Molecular Formula | H2O |
| Bond Angle | 104.5deg |
| Polarity | Polar molecule |
| Hydrogen Bonds | Strong intermolecular forces |
| Physical State | Liquid at room temperature |
Famous Chemists and Their Discoveries
Chemistry has been shaped by the groundbreaking work of famous chemists whose discoveries transformed science and industry. Their innovations laid the foundation for modern chemical understanding and technological advancement.
Marie Curie pioneered research on radioactivity, discovering polonium and radium, which advanced nuclear chemistry. Dmitri Mendeleev created the Periodic Table, organizing elements by atomic weight and properties, predicting undiscovered elements.
