Atomic theory explains the fundamental structure and behavior of matter by describing atoms as the basic building blocks. It traces the historical development from ancient philosophical ideas to modern scientific understanding supported by experimental evidence. Visualizing this progression through an infographic highlights key discoveries and concepts that shape contemporary chemistry and physics.
The Evolution of Atomic Theory
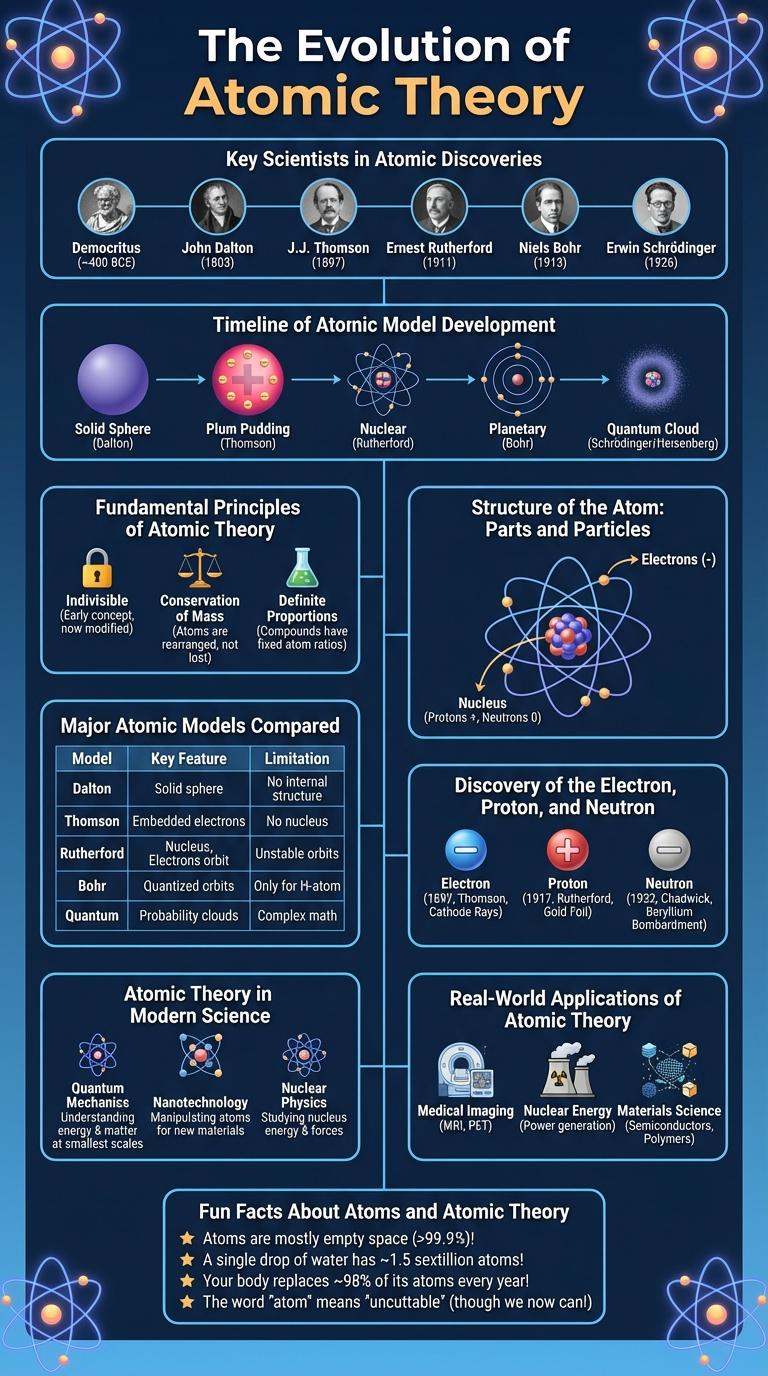
The Evolution of Atomic Theory traces the development of scientific understanding about the atom, from ancient philosophical ideas to modern quantum mechanics. Early models by Dalton, Thomson, and Rutherford progressively revealed the atom's structure, leading to Bohr's planetary model and Schrodinger's quantum mechanical model. This progression highlights key discoveries such as the electron, nucleus, and atomic orbitals that define current atomic theory.
| Scientist | Contribution |
|---|---|
| John Dalton | Proposed the atom as an indivisible particle. |
| J.J. Thomson | Discovered the electron and developed the plum pudding model. |
| Ernest Rutherford | Identified the nucleus through gold foil experiment. |
| Niels Bohr | Developed the planetary model of the atom with electron orbits. |
| Erwin Schrodinger | Formulated the quantum mechanical model of atomic orbitals. |
Key Scientists in Atomic Discoveries
The development of atomic theory marks a pivotal chapter in science, revealing the fundamental nature of matter. Key scientists contributed unique insights that collectively shaped our understanding of atoms.
John Dalton proposed the first modern atomic theory in the early 19th century, stating that atoms are indivisible and each element consists of identical atoms. Dmitri Mendeleev's periodic table arranged elements by atomic mass, highlighting periodic properties linked to atomic structure.
J.J. Thomson discovered the electron in 1897, introducing the concept of subatomic particles and the "plum pudding" model of the atom. Ernest Rutherford's gold foil experiment revealed the nucleus, demonstrating that atoms have a dense positive core surrounded by electrons.
Niels Bohr enhanced atomic theory by proposing quantized electron orbits, explaining atomic emission spectra and chemical behavior. James Chadwick discovered the neutron in 1932, completing the nuclear model with protons and neutrons in the nucleus.
Timeline of Atomic Model Development
| Year | Key Development |
|---|---|
| 1803 | John Dalton proposes the first atomic theory describing atoms as indivisible particles. |
| 1897 | J.J. Thomson discovers the electron and introduces the Plum Pudding Model. |
| 1911 | Ernest Rutherford presents the nuclear model with a dense atomic nucleus. |
| 1913 | Niels Bohr develops the Bohr Model featuring quantized electron orbits. |
| 1926 | Erwin Schrodinger formulates the quantum mechanical model using wave functions. |
Fundamental Principles of Atomic Theory
The atomic theory explains the nature and behavior of atoms, the basic building blocks of matter. This infographic highlights the fundamental principles that form the foundation of atomic theory.
- All matter is composed of atoms - Atoms are indivisible particles that constitute all elements and compounds.
- Atoms of the same element are identical - Atoms in a given element have the same size, mass, and chemical properties.
- Atoms combine in fixed ratios - Chemical compounds form when atoms bond in specific whole-number ratios.
Structure of the Atom: Parts and Particles
The atomic theory explains that atoms are the basic units of matter, consisting of a nucleus and surrounding electrons. The nucleus contains protons, which have a positive charge, and neutrons, which are neutral particles. Electrons, negatively charged, orbit the nucleus in energy levels, creating the overall structure of the atom.
Major Atomic Models Compared
What are the major atomic models and how do they differ? The atomic theory has evolved through several key models including Dalton's, Thomson's, Rutherford's, Bohr's, and the Quantum Mechanical model. Each model introduced new concepts about atomic structure, from indivisible particles to electron probability clouds.
Discovery of the Electron, Proton, and Neutron
The atomic theory has evolved through significant discoveries of subatomic particles that define the structure of the atom. Key findings include the identification of the electron, proton, and neutron, each contributing to modern atomic models.
The discovery of these particles transformed scientific understanding by revealing the complex nature of atomic components beyond a simple indivisible unit.
- Electron Discovery - J.J. Thomson identified the electron in 1897 through cathode ray tube experiments, revealing negatively charged particles within the atom.
- Proton Discovery - Ernest Rutherford discovered the proton in 1917 by analyzing hydrogen nuclei, establishing the presence of positively charged particles in the nucleus.
- Neutron Discovery - James Chadwick confirmed the neutron in 1932 through nuclear reaction experiments, identifying the neutral particle essential to atomic mass and stability.
Atomic Theory in Modern Science
Atomic theory forms the foundation of modern chemistry and physics, explaining the nature and behavior of matter. Advances in atomic theory continue to drive innovations in technology, medicine, and materials science.
- Quantum Mechanics - Describes electrons as wave-particles existing in probabilistic orbitals rather than fixed paths.
- Subatomic Particles - Atoms consist of protons, neutrons, and electrons, with quarks forming protons and neutrons.
- Atomic Models - The Bohr model was refined into the quantum mechanical model to better predict atomic behavior.
Modern atomic theory integrates experimental evidence from spectroscopy, particle physics, and quantum chemistry to explain atomic structure and interactions.
Real-World Applications of Atomic Theory
Atomic theory explains the structure and behavior of atoms, the fundamental building blocks of matter. Understanding atoms allows scientists to develop innovative technologies and improve various industries.
In medicine, atomic theory enables advanced imaging techniques like MRI and PET scans, enhancing diagnostics and treatment. In energy, it underpins nuclear power generation, providing a significant source of electricity. Materials science relies on atomic models to design stronger, lighter materials for aerospace and electronics.
