Infographics about elements provide a visually engaging way to understand the building blocks of matter. They highlight key properties, atomic numbers, and classifications such as metals, nonmetals, and metalloids. These visuals simplify complex scientific data into easily digestible information for learners and enthusiasts alike.
Essential Elements of the Periodic Table
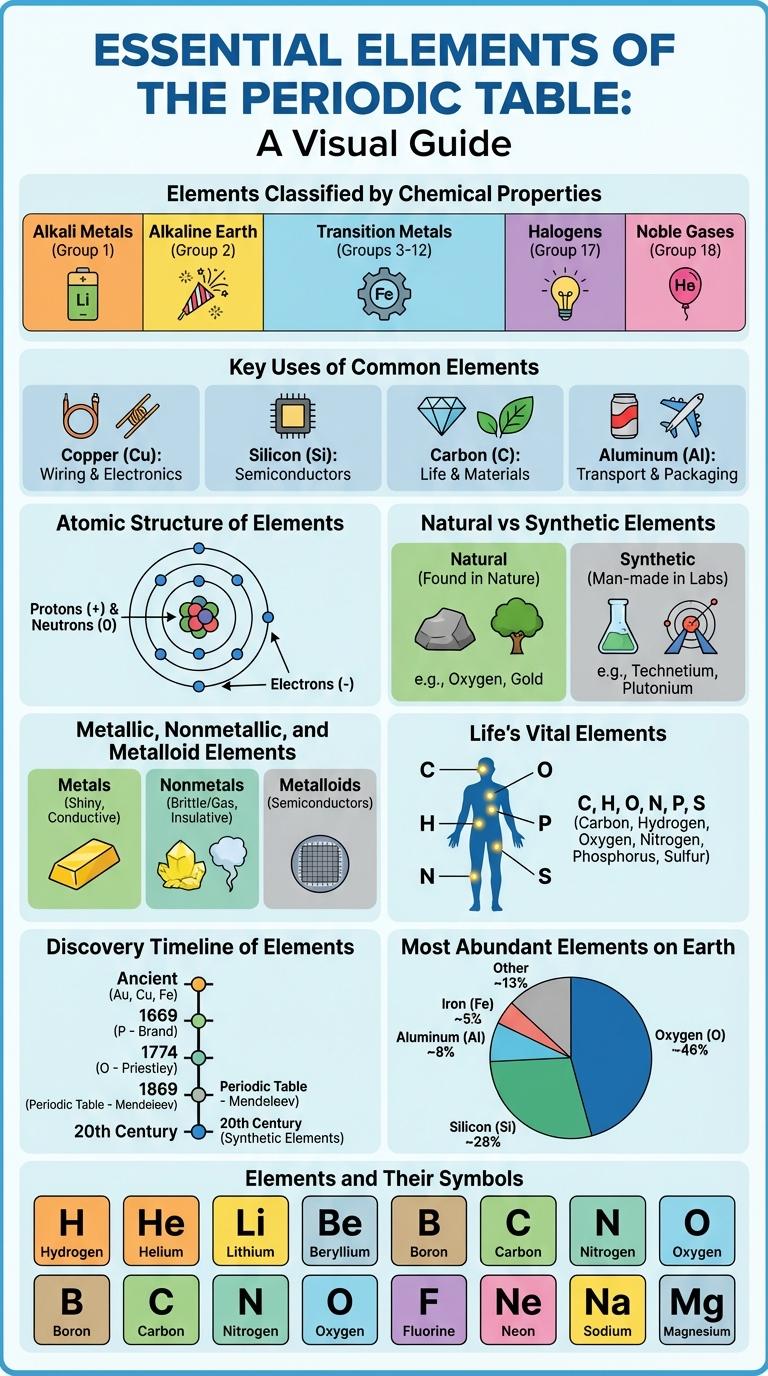
The periodic table organizes all known chemical elements based on their atomic number, electron configuration, and recurring chemical properties. Essential elements are fundamental building blocks in chemistry and materials science.
- Hydrogen - The lightest element, hydrogen is essential for water formation and organic compounds.
- Carbon - Known as the backbone of life, carbon forms a vast variety of complex molecules.
- Oxygen - A critical component of the Earth's atmosphere, oxygen supports respiration and combustion.
Elements Classified by Chemical Properties
Elements can be classified based on their chemical properties into metals, nonmetals, and metalloids. These categories help in understanding their behavior in reactions and physical characteristics.
Metals are typically shiny, conductive, and malleable, making them essential in construction and electronics. Nonmetals vary widely but generally have poor conductivity and are found in gases or brittle solids at room temperature. Metalloids exhibit mixed properties, useful in semiconductors and various chemical processes.
Key Uses of Common Elements
Elements play a crucial role in various industries and everyday life. Understanding their key uses highlights their importance in technology, health, and manufacturing.
- Hydrogen - Used primarily in fuel cells and as a clean energy source for transportation.
- Carbon - Essential in organic chemistry, carbon forms the backbone of life and is used in steel production.
- Oxygen - Vital for respiration and widely used in medical treatments and industrial processes.
- Iron - The main component of steel, iron is fundamental for construction and manufacturing.
- Silicon - Key material in electronics, especially in semiconductors and solar panels.
These elements demonstrate a diverse range of applications that support modern advancements and daily functions.
Atomic Structure of Elements
Natural vs Synthetic Elements
Elements can be classified into natural and synthetic categories based on their origin. Understanding the differences between these types reveals their roles in science and industry.
- Natural Elements - Occur naturally in Earth's crust, atmosphere, and oceans without human intervention.
- Synthetic Elements - Man-made elements created in laboratories through nuclear reactions or particle accelerators.
- Stability - Natural elements tend to be more stable, while synthetic elements often have short half-lives and decay rapidly.
Metallic, Nonmetallic, and Metalloid Elements
Elements are classified into three main categories: metallic, nonmetallic, and metalloid. Each category exhibits distinct physical and chemical properties essential for various applications.
Metallic elements are good conductors of heat and electricity, have high melting points, and are usually malleable and ductile. Nonmetallic elements lack these properties and are often poor conductors, with diverse states including gases and solids.
Metalloids possess properties intermediate between metals and nonmetals, making them useful in semiconductors and other technologies. Their behavior varies depending on environmental conditions and specific element characteristics.
Life's Vital Elements
Life's vital elements include carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur, essential for biological molecules. These elements form the building blocks of proteins, nucleic acids, carbohydrates, and lipids. Understanding their roles helps explain how life sustains and evolves on Earth.
Discovery Timeline of Elements
The Discovery Timeline of Elements traces the identification of chemical elements from ancient times to the modern era. Early elements like gold and silver were known since antiquity, while many more were discovered through scientific advancements.
In the 18th and 19th centuries, chemists like Antoine Lavoisier and Dmitri Mendeleev contributed to element classification and discovery. Modern technologies continue to reveal synthetic elements beyond uranium in the periodic table.
Most Abundant Elements on Earth
What are the most abundant elements found on Earth? Oxygen and silicon are the two most abundant elements, making up a significant portion of the Earth's crust. Oxygen accounts for about 46.6%, while silicon makes up approximately 27.7%.
| Element | Abundance in Earth's Crust (%) |
|---|---|
| Oxygen (O) | 46.6% |
| Silicon (Si) | 27.7% |
| Aluminum (Al) | 8.1% |
| Iron (Fe) | 5.0% |
| Calcium (Ca) | 3.6% |
How do these elements contribute to the composition of Earth? Oxygen bonds with silicon to form silicate minerals, which are the building blocks of most rocks. Metals such as aluminum, iron, and calcium provide structural strength and are essential to Earth's geology and mineral diversity.
