Isotopes are variants of the same chemical element that differ in neutron number, affecting atomic mass without changing chemical properties. Infographics can visually simplify the comparison of isotopes by highlighting their atomic structure, stability, and common applications. Understanding isotopes is crucial for fields ranging from medicine to environmental science.
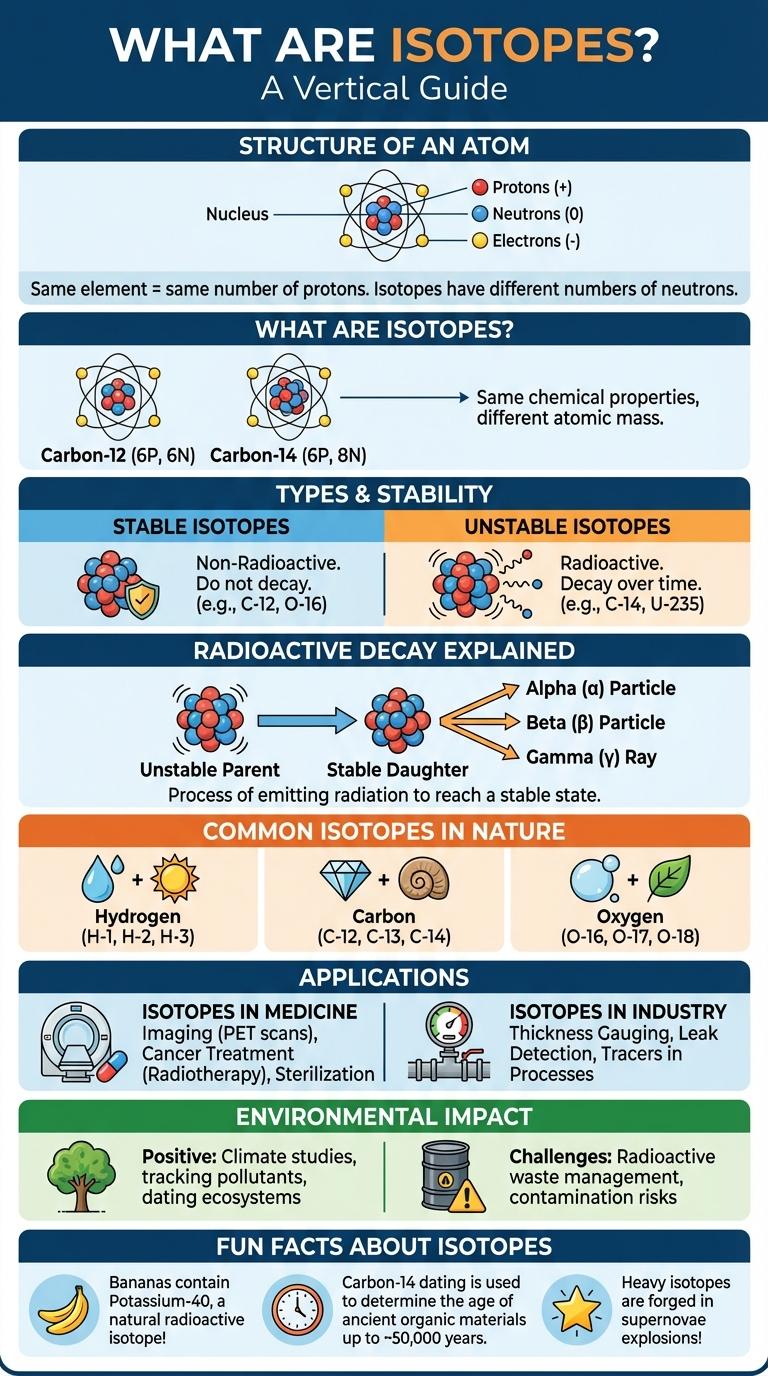
What Are Isotopes?
Isotopes are variants of the same chemical element that differ in neutron number, resulting in different atomic masses. These variations occur naturally and can influence the stability and decay properties of the element.
- Same proton number - Isotopes share the same number of protons, defining the element.
- Different neutron count - Neutron numbers vary, creating isotopic differences.
- Varied stability - Some isotopes are stable while others are radioactive.
Isotopes have important applications in fields like medicine, archaeology, and environmental science due to their unique properties.
Structure of an Atom
An atom consists of a nucleus containing protons and neutrons, surrounded by electrons in orbitals. Isotopes have the same number of protons but differ in neutron count, affecting atomic mass. These variations influence nuclear stability and atomic behavior without changing chemical properties.
Types of Isotopes
Isotopes are atoms of the same element with different numbers of neutrons, resulting in varied atomic masses. Types of isotopes include stable isotopes, which do not change over time, and radioactive isotopes, which decay and emit radiation. These differences impact their applications in fields like medicine, archaeology, and environmental science.
Stable vs. Unstable Isotopes
Isotopes are variants of elements differing in neutron number but sharing the same proton count. Stable isotopes maintain consistent nuclei without undergoing radioactive decay.
Unstable isotopes, or radioisotopes, emit radiation as they transform into more stable forms. Their decay rates are quantified by half-lives, ranging from fractions of a second to millions of years.
Common Isotopes in Nature
What are common isotopes found in nature? Natural isotopes are variants of elements with differing neutron numbers, occurring naturally in the environment. Examples include Carbon-12, Oxygen-16, and Uranium-238, which have significant roles in scientific research and daily life.
Isotopes in Medicine
Isotopes play a crucial role in medical diagnostics and treatment, particularly through radioactive isotopes used in imaging and therapy. These isotopes help visualize internal organs and target diseased cells with precision.
Commonly used isotopes in medicine include Technetium-99m for imaging and Iodine-131 for treating thyroid conditions. Their unique properties enable non-invasive diagnosis and effective cancer treatments.
Isotopes in Industry
Isotopes play a crucial role in various industrial applications, enhancing efficiency and safety. Their unique properties enable innovations in fields such as energy, manufacturing, and quality control.
- Radiography and Non-Destructive Testing - Isotopes like Cobalt-60 emit gamma rays used to inspect metal parts and welds without damaging the material.
- Oil and Gas Exploration - Noble gas isotopes such as Helium-3 assist in tracing and analyzing reservoirs for improved extraction methods.
- Material Thickness Gauging - Beta-emitting isotopes, including Strontium-90, measure material thickness in production lines accurately.
Radioactive Decay Explained
Isotopes are atoms of the same element with different numbers of neutrons, resulting in varied atomic masses. Some isotopes are stable, while others are radioactive and undergo decay over time.
Radioactive decay is the process by which unstable isotopes release energy by emitting particles or electromagnetic waves. This transformation changes the isotope into a different element or a more stable form. The decay rate is measured by the isotope's half-life, which indicates the time for half of the sample to decay.
Environmental Impact of Isotopes
| Isotope | Environmental Impact |
|---|---|
| Carbon-14 | Used in dating organic materials, its radioactive decay has minimal direct environmental risk but helps track climate change effects. |
| Uranium-238 | Radioactive decay contributes to soil and water contamination near mining sites, causing long-term ecological damage. |
| Deuterium (Hydrogen-2) | Stable isotope used in water tracing to study pollution and water cycle without environmental toxicity. |
| Strontium-90 | Byproduct of nuclear fission, it accumulates in bones of living organisms, leading to genetic mutations and ecosystem imbalance. |
| Radon-222 | Radioactive gas from uranium decay, it seeps into buildings causing indoor air pollution and health hazards. |
