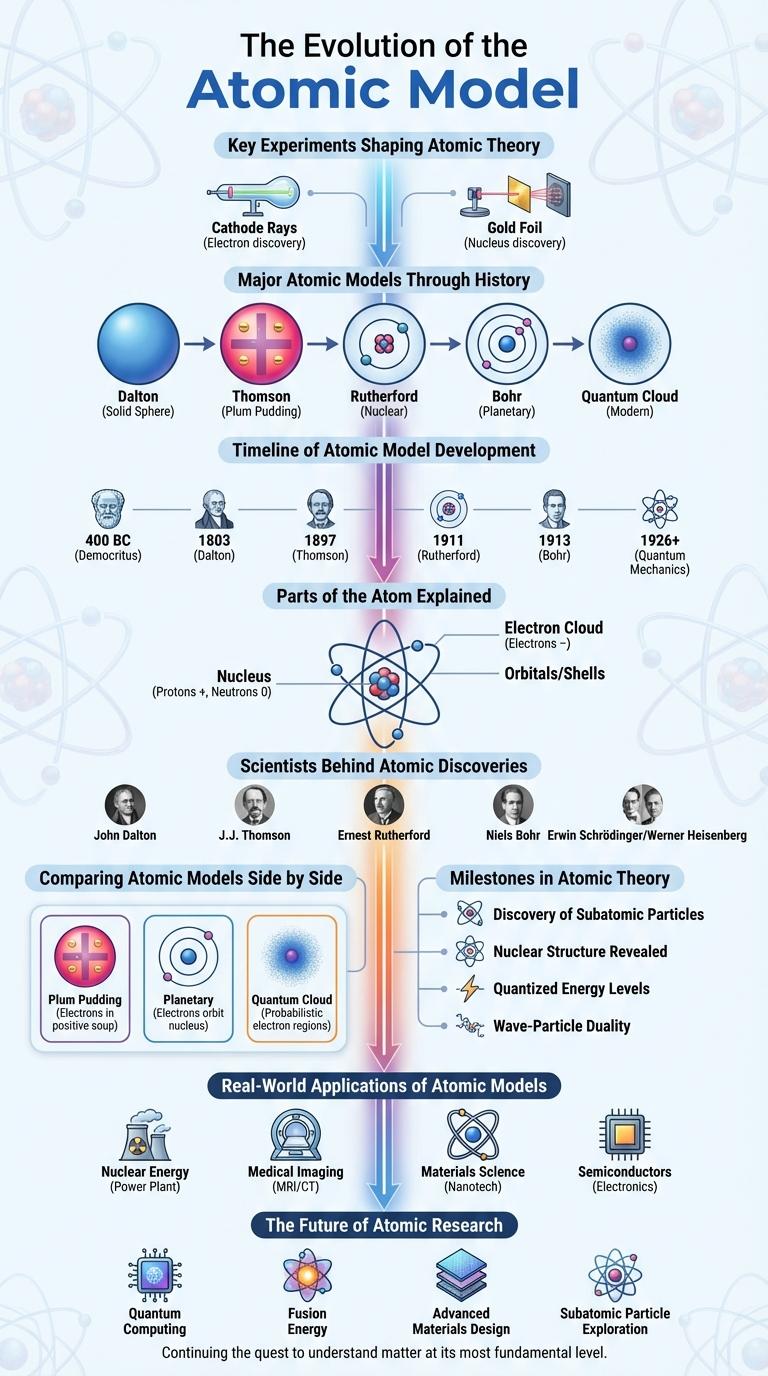
The atomic model infographic visually represents the evolution of atomic theory from Dalton's solid sphere model to the quantum mechanical model. It highlights key scientists, discoveries, and the structure of atoms, including protons, neutrons, and electrons. This infographic provides a clear understanding of atomic concepts essential for studying chemistry and physics.
The Evolution of the Atomic Model
The atomic model has undergone significant changes over time as scientific understanding has progressed. Each stage of its evolution refined the concepts of atomic structure and behavior.
The evolution of the atomic model highlights key discoveries from early theories to modern quantum mechanics.
- Dalton's Atomic Theory - Proposed atoms as indivisible particles forming all matter in the early 19th century.
- Thomson's Plum Pudding Model - Introduced electrons embedded in a positively charged sphere in 1904.
- Rutherford's Nuclear Model - Discovered the nucleus, showing atoms have a dense central core in 1911.
- Bohr Model - Described electrons orbiting the nucleus in defined energy levels in 1913.
- Quantum Mechanical Model - Represents electrons as probabilistic clouds based on wave-particle duality and quantum theory, developed mid-20th century.
Key Experiments Shaping Atomic Theory
The atomic model has evolved through critical experiments that reveal the structure of matter. These experiments laid the foundation for modern atomic theory by uncovering the nature of atoms and subatomic particles.
Key experiments shaped our understanding of atomic structure and behavior.
- Dalton's Atomic Theory - Proposed atoms as indivisible particles and elements made of identical atoms in 1803.
- Thomson's Cathode Ray Experiment - Discovered electrons and suggested atoms contain negative charges in 1897.
- Rutherford's Gold Foil Experiment - Revealed the atomic nucleus as a dense, positively charged center in 1911.
- Bohr's Model - Introduced quantized electron orbits explaining atomic emission spectra in 1913.
- Chadwick's Neutron Discovery - Identified neutrons as neutral particles within the nucleus in 1932.
Major Atomic Models Through History
The atomic model has evolved significantly from early philosophical ideas to advanced scientific theories. Each major model contributed to a deeper understanding of atomic structure and behavior.
Dalton's atomic model introduced the concept of atoms as indivisible particles in the early 19th century. Thomson's plum pudding model later proposed atoms as spheres with embedded electrons.
Rutherford's nuclear model revealed a dense, positively charged nucleus surrounded by electrons in 1911. Bohr's model refined this with electrons orbiting the nucleus in fixed energy levels.
Quantum mechanical models, developed in the 20th century, described electron clouds and probabilities rather than fixed orbits. These models remain the foundation of modern atomic theory and chemistry.
Timeline of Atomic Model Development
The timeline of atomic model development begins with Dalton's solid sphere model in 1803, which introduced the idea of indivisible atoms. Thomson's plum pudding model in 1897 discovered electrons embedded in a positive sphere, shifting atomic understanding. Rutherford's nuclear model in 1911 revealed a dense nucleus, followed by Bohr's planetary orbit model in 1913 that described quantized electron orbits.
Parts of the Atom Explained
The atom consists of three main parts: protons, neutrons, and electrons. Protons and neutrons form the nucleus at the center, while electrons orbit the nucleus in energy levels. The number of protons defines the element, and electrons determine chemical properties.
Scientists Behind Atomic Discoveries
| Scientist | Key Contribution to Atomic Model |
|---|---|
| John Dalton (1803) | Proposed the first atomic theory, describing atoms as indivisible particles. |
| J.J. Thomson (1897) | Discovered the electron; developed the plum pudding model of the atom. |
| Ernest Rutherford (1911) | Conducted gold foil experiment; proposed the nuclear model with a dense nucleus. |
| Niels Bohr (1913) | Introduced quantized electron orbits; explained atomic emission spectra. |
| Erwin Schrodinger (1926) | Formulated wave equation; established quantum mechanical model of the atom. |
Comparing Atomic Models Side by Side
How do different atomic models compare in explaining atomic structure?
Atomic models have evolved from simple to complex representations. Each model provides unique insights into atomic behavior and structure.
| Atomic Model | Key Features |
|---|---|
| Dalton's Model | Atoms as solid, indivisible spheres. |
| Thomson's Model | "Plum pudding" structure with electrons embedded in a positive matrix. |
| Rutherford's Model | Small, dense nucleus surrounded by electrons in mostly empty space. |
| Bohr's Model | Electrons orbit nucleus in fixed energy levels. |
| Quantum Mechanical Model | Electron probabilities and wave functions define atomic orbitals. |
Milestones in Atomic Theory
The atomic model has evolved significantly through key scientific milestones. Each advancement enhanced the understanding of atomic structure and behavior.
John Dalton proposed the first atomic theory in the early 19th century, describing atoms as indivisible particles. J.J. Thomson discovered the electron in 1897, introducing the plum pudding model. Ernest Rutherford's gold foil experiment in 1911 revealed the nuclear atom with a dense central nucleus.
Real-World Applications of Atomic Models
Atomic models have revolutionized science by providing a framework to understand the structure and behavior of atoms. These models underpin modern technologies and innovations across various industries.
In medicine, atomic models guide the development of diagnostic tools like MRI and radiation therapy for cancer treatment. In electronics, they help design semiconductors and transistors that form the backbone of computers and smartphones.
