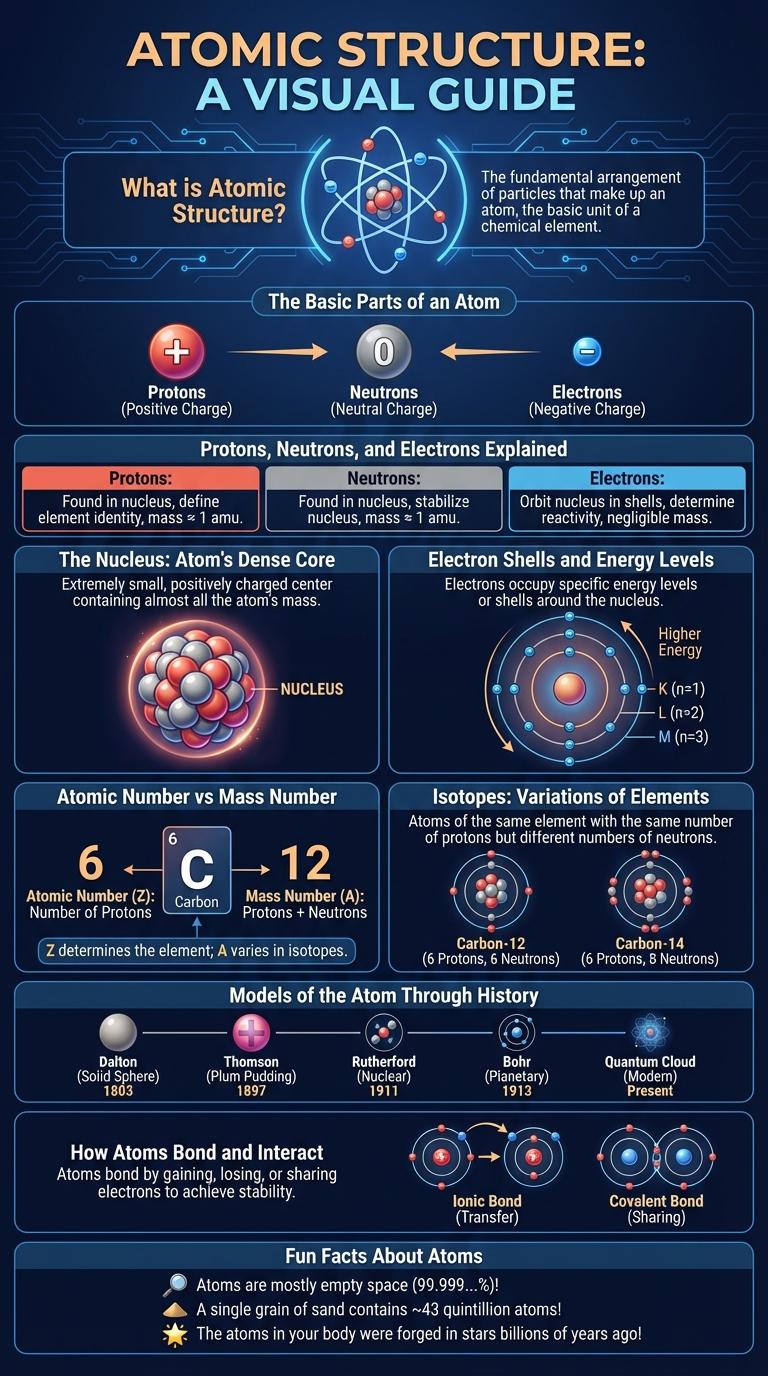
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in defined energy levels or shells. This infographic visually breaks down the components of atomic structure, highlighting the arrangement and function of each particle within the atom. Understanding atomic structure is essential for comprehending chemical behavior and physical properties of matter.
What is Atomic Structure?
Atomic structure refers to the arrangement of subatomic particles within an atom. It consists of a nucleus containing protons and neutrons, surrounded by electrons in defined energy levels. This structure determines the chemical properties and behavior of an element.
The Basic Parts of an Atom
What are the basic parts of an atom?
An atom consists of three main particles: protons, neutrons, and electrons. Protons and neutrons form the nucleus, while electrons orbit around it.
Protons, Neutrons, and Electrons Explained
The atomic structure consists of three main subatomic particles: protons, neutrons, and electrons. Protons and neutrons form the nucleus, while electrons orbit around this central core.
Protons carry a positive charge and determine the element's identity due to their fixed number in each atom. Neutrons have no charge and contribute to the atom's mass and stability by holding the nucleus together.
The Nucleus: Atom's Dense Core
The nucleus is the dense central core of an atom, containing protons and neutrons. It holds nearly all of the atom's mass while occupying a tiny fraction of its volume.
- Protons - Positively charged particles that determine the atomic number and element identity.
- Neutrons - Neutral particles that contribute to atomic mass and influence isotope stability.
- Nuclear Forces - Strong interactions that hold protons and neutrons tightly bound within the nucleus.
The nucleus plays a crucial role in nuclear reactions and atomic stability.
Electron Shells and Energy Levels
The atomic structure consists of a nucleus surrounded by electron shells that hold electrons in quantized energy levels. Electron shells represent fixed distances from the nucleus where electrons orbit with specific energy values.
- Electron Shells - Electron shells are layers around the nucleus where electrons are found, each shell corresponding to a principal energy level.
- Energy Levels - Energy levels define the amount of energy electrons possess in their shells, increasing as shells are farther from the nucleus.
- Shell Capacity - Each electron shell holds a maximum number of electrons determined by the formula 2n2, where n is the shell number.
Atomic Number vs Mass Number
The atomic structure consists of protons, neutrons, and electrons. The atomic number and mass number are key properties that define an element's identity and mass.
The atomic number represents the number of protons in the nucleus. The mass number is the total count of protons and neutrons. Elements with the same atomic number have identical chemical properties but can differ in mass number due to varying neutrons.
Isotopes: Variations of Elements
Isotopes are variations of elements that differ in the number of neutrons within their atomic nuclei. These differences in neutron count result in atoms with the same number of protons but varying atomic masses. Understanding isotopes is crucial for applications in medicine, archaeology, and nuclear energy.
Models of the Atom Through History
| Model | Description |
|---|---|
| Dalton's Atomic Model (1803) | Proposed that atoms are indivisible, solid spheres representing elements with unique weights. |
| Thomson's Plum Pudding Model (1897) | Described atoms as spheres of positive charge with embedded electrons, resembling a "plum pudding." |
| Rutherford's Nuclear Model (1911) | Introduced a dense, positively charged nucleus surrounded by electrons orbiting at a distance. |
| Bohr's Model (1913) | Depicted electrons orbiting the nucleus in fixed energy levels or shells. |
| Quantum Mechanical Model (1926) | Described electrons as wave-like particles with uncertain positions, occupying probabilistic orbitals. |
How Atoms Bond and Interact
Atoms bond and interact through their electrons to form molecules and compounds. The nature of these interactions determines the physical and chemical properties of substances.
Understanding atomic bonding explains chemical reactions and material behavior.
- Covalent Bonding - Atoms share electron pairs to achieve full outer electron shells, creating strong bonds.
- Ionic Bonding - Atoms transfer electrons, resulting in positively and negatively charged ions that attract each other.
- Metallic Bonding - Electrons move freely among metal atoms, allowing conductivity and malleability.
