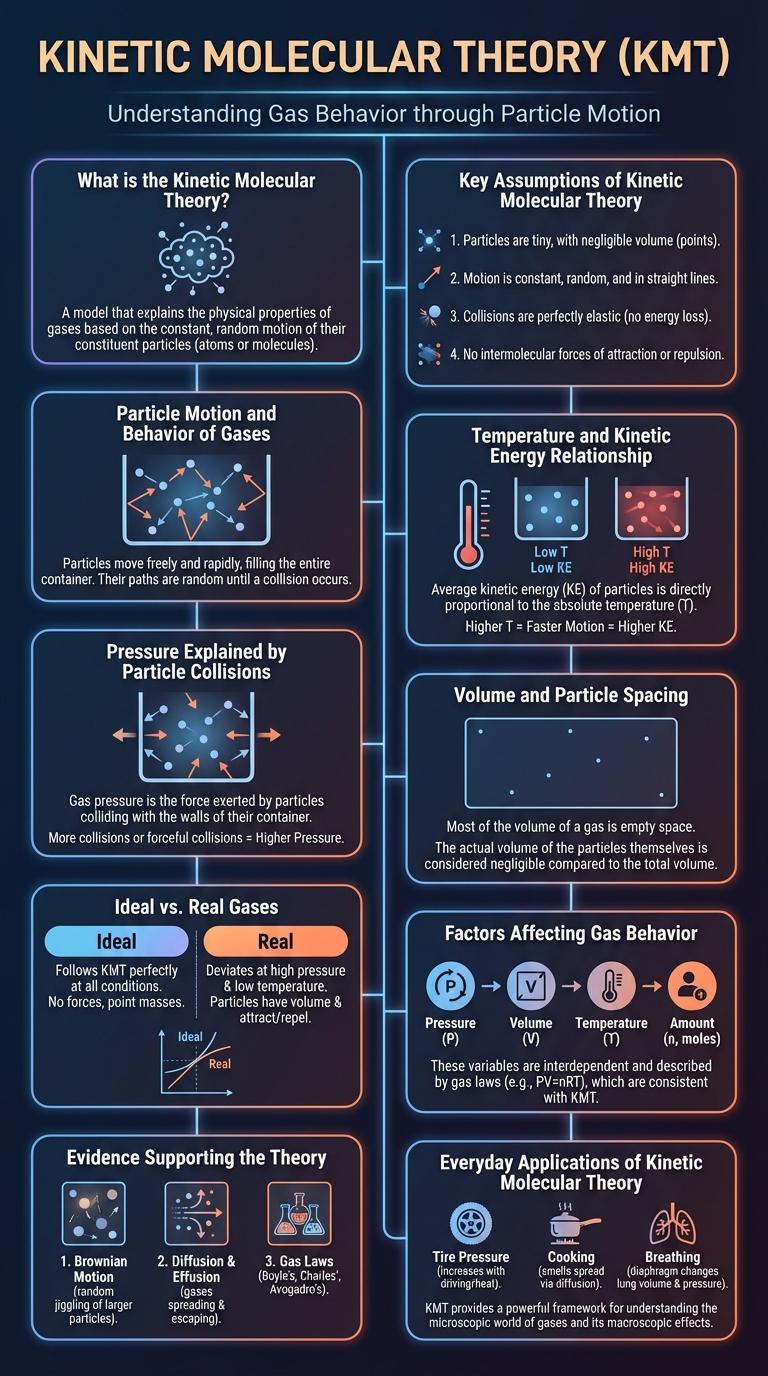
The kinetic molecular theory explains the behavior of gases by describing particles in constant, random motion. It highlights how temperature, pressure, and volume influence particle movement and energy. This infographic visually breaks down these principles to enhance understanding of gas properties.
What is the Kinetic Molecular Theory?
The Kinetic Molecular Theory explains the behavior of gas particles in terms of their motion. It states that gas particles are in constant, random motion and collide elastically with each other and the walls of their container. This theory helps describe properties such as pressure, temperature, and volume in gases.
Key Assumptions of Kinetic Molecular Theory
The Kinetic Molecular Theory explains the behavior of gases by describing particles in constant motion. It focuses on the energy and movement of gas molecules to explain gas properties.
Key assumptions include that gas particles are tiny, hard spheres with negligible volume. They move randomly in straight lines, colliding elastically without energy loss.
Particle Motion and Behavior of Gases
The Kinetic Molecular Theory explains the behavior of gases by describing particle motion and energy. Gas particles are in constant, random motion, colliding elastically with each other and container walls.
- Particle Motion - Gas particles move rapidly and randomly in all directions.
- Energy Distribution - Particle kinetic energy varies but is proportional to temperature.
- Collisions - Collisions between particles and container walls are perfectly elastic, causing pressure.
Temperature and Kinetic Energy Relationship
| Concept | Description |
|---|---|
| Kinetic Molecular Theory | Explains the behavior of particles in gases, stating that particles are in constant, random motion. |
| Temperature | Measures the average kinetic energy of gas particles; higher temperature means higher energy. |
| Kinetic Energy | Energy that particles possess due to their motion; directly proportional to temperature in Kelvin. |
| Relationship | As temperature increases, the kinetic energy of particles rises, causing faster particle movement. |
| Practical Implication | Increased temperature leads to higher pressure if volume is constant, due to more frequent and forceful collisions. |
Pressure Explained by Particle Collisions
The kinetic molecular theory explains how gas particles behave and interact. Pressure results from collisions of these particles with container walls.
- Particle Motion - Gas particles move randomly at high speeds in all directions.
- Collision Force - Particles collide elastically with container walls, exerting force.
- Pressure Origin - Pressure is caused by the collective impact of countless particle collisions on surfaces.
Understanding particle collisions helps explain gas pressure variations with temperature and volume changes.
Volume and Particle Spacing
Kinetic Molecular Theory explains the behavior of particles in different states of matter. Volume and particle spacing are key concepts that describe how particles move and interact.
In gases, particles are widely spaced and move rapidly, filling the entire volume of their container. Liquids have closer particle spacing, allowing particles to slide past one another while maintaining a fixed volume. Solids exhibit tightly packed particles with minimal movement, resulting in a definite volume and shape.
Ideal vs. Real Gases
The Kinetic Molecular Theory explains the behavior of gas particles in motion. It describes gases as collections of small particles moving in constant, random motion.
Ideal gases perfectly follow this theory, with no intermolecular forces and elastic collisions. Real gases deviate from ideal behavior due to particle volume and attractive forces.
Factors Affecting Gas Behavior
The kinetic molecular theory explains gas behavior based on particle motion and energy. Factors affecting gas behavior include temperature, pressure, and volume, which influence particle speed and collision frequency. These variables determine how gases expand, compress, and exert pressure in different conditions.
Evidence Supporting the Theory
What evidence supports the kinetic molecular theory? The theory is supported by observable phenomena like gas pressure and temperature changes, which relate to particle motion. Experiments using Brownian motion visually confirm that particles move constantly and randomly, validating the theory's core principles.
