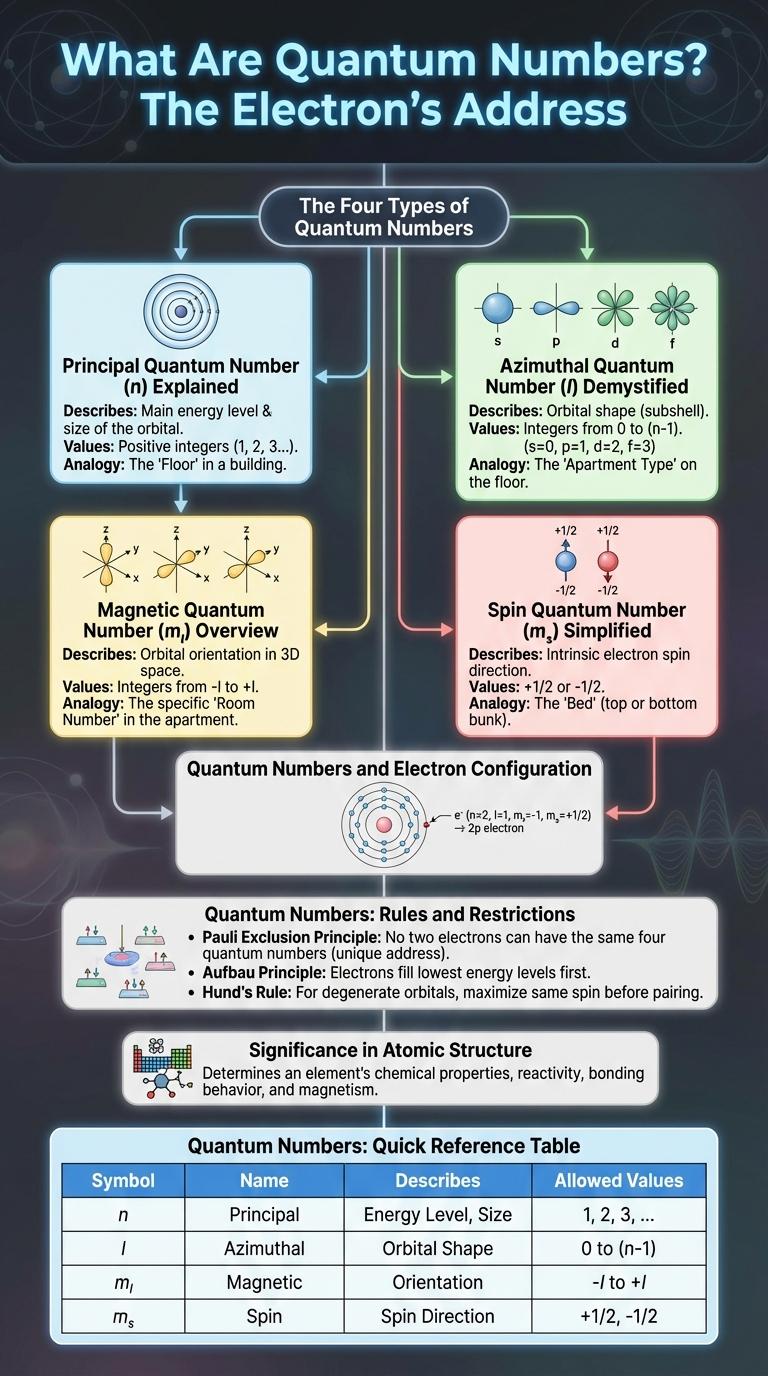
Quantum numbers define the unique quantum state of an electron within an atom, specifying its energy level, angular momentum, magnetic orientation, and spin direction. These values are essential for understanding electron configurations and predicting chemical behavior. Visualizing quantum numbers through an infographic simplifies complex quantum concepts for easier learning and retention.
What Are Quantum Numbers?
| Quantum Numbers | Description |
|---|---|
| Principal Quantum Number (n) | Determines the energy level and size of an orbital. Values are positive integers starting from 1. |
| Azimuthal Quantum Number (l) | Defines the shape of the orbital. It ranges from 0 to (n-1) for each energy level. |
| Magnetic Quantum Number (ml) | Specifies the orientation of the orbital in space. Values range from -l to +l, including zero. |
| Spin Quantum Number (ms) | Describes the electron's spin direction. Possible values are +1/2 or -1/2. |
The Four Types of Quantum Numbers
Quantum numbers describe the unique quantum state of electrons in an atom. These numbers determine the electron's energy, shape, orientation, and spin properties.
- Principal Quantum Number (n) - Indicates the main energy level or shell of an electron.
- Azimuthal Quantum Number (l) - Defines the shape of the electron's orbital within a given energy level.
- Magnetic Quantum Number (ml) - Specifies the orientation of the orbital in space relative to an external magnetic field.
- Spin Quantum Number (ms) - Represents the intrinsic spin direction of the electron, either + 1/2 or - 1/2.
Each combination of these four quantum numbers uniquely identifies an electron in an atom.
Principal Quantum Number (n) Explained
The Principal Quantum Number (n) determines the main energy level or shell of an electron in an atom, ranging from 1 to infinity. It influences the size and energy of the electron cloud, with higher values indicating electrons farther from the nucleus and higher energy states. This quantum number plays a critical role in the electronic configuration and chemical properties of elements.
Azimuthal Quantum Number (l) Demystified
What is the Azimuthal Quantum Number and why is it important in quantum mechanics? The Azimuthal Quantum Number, symbolized as l, determines the shape of an electron's orbital within an atom. It plays a crucial role in defining the subshell and influences the energy levels and chemical properties of elements.
| Azimuthal Quantum Number (l) | Description |
|---|---|
| Values | Integer values from 0 to n-1, where n is the principal quantum number |
| Orbital Shapes | l = 0 (s), l = 1 (p), l = 2 (d), l = 3 (f) |
| Subshells | Defines subshell type, impacting electron distribution |
| Energy Influence | Contributes to energy splitting within the same principal level |
Magnetic Quantum Number (ml) Overview
The Magnetic Quantum Number (ml) specifies the orientation of an electron's orbital within a given subshell. It is an integer value ranging from -l to +l, where l is the azimuthal quantum number.
The value of ml determines the number of orbitals available in a particular subshell and their spatial orientation. For example, when l = 1 (p-subshell), ml can be -1, 0, or +1, corresponding to three distinct p orbitals. This quantum number is essential for understanding electron configuration and magnetic properties of atoms.
Spin Quantum Number (ms) Simplified
The Spin Quantum Number (ms) represents the intrinsic angular momentum of an electron. It indicates the direction of the electron's spin, which can have only two possible values.
These values are +1/2 and -1/2, signifying spin "up" and spin "down" respectively. This property is fundamental in defining the electron's magnetic moment and behavior in an atom.
Quantum Numbers and Electron Configuration
Quantum numbers describe the properties of atomic orbitals and the electrons in those orbitals, essential for understanding electron configuration. There are four quantum numbers: principal (n), angular momentum (l), magnetic (m_l), and spin (m_s), each representing different electron characteristics. Electron configuration uses quantum numbers to determine the arrangement of electrons in atoms, influencing chemical behavior and bonding.
Quantum Numbers: Rules and Restrictions
Quantum numbers define the unique quantum state of an electron in an atom. They follow strict rules and restrictions to maintain the Pauli exclusion principle and electron configuration consistency.
- Principal Quantum Number (n) - Determines the energy level and size of the electron orbital, only positive integers (1, 2, 3...).
- Azimuthal Quantum Number (l) - Defines the orbital shape and ranges from 0 to n-1 for each principal quantum number.
- Magnetic Quantum Number (ml) - Specifies the orientation of the orbital, ranging from -l to +l, including zero.
- Spin Quantum Number (ms) - Represents the electron spin, restricted to +1/2 or -1/2 values.
- Pauli Exclusion Principle - No two electrons in an atom can have the same set of all four quantum numbers.
Significance in Atomic Structure
Quantum numbers define the unique quantum state of an electron in an atom, describing its energy, angular momentum, magnetic orientation, and spin. These values are essential for predicting electron configurations and chemical behavior.
The principal quantum number (n) indicates the electron's energy level and distance from the nucleus. Other quantum numbers determine shape, orientation, and spin, influencing atomic structure and reactivity.
