The periodic table organizes chemical elements based on their atomic number, electron configurations, and recurring chemical properties, serving as a fundamental tool in chemistry. This infographic visually highlights the arrangement, classification, and unique characteristics of each element, improving comprehension and retention. Interactive elements within the design facilitate deeper exploration of element families, atomic mass, and trends across periods and groups.
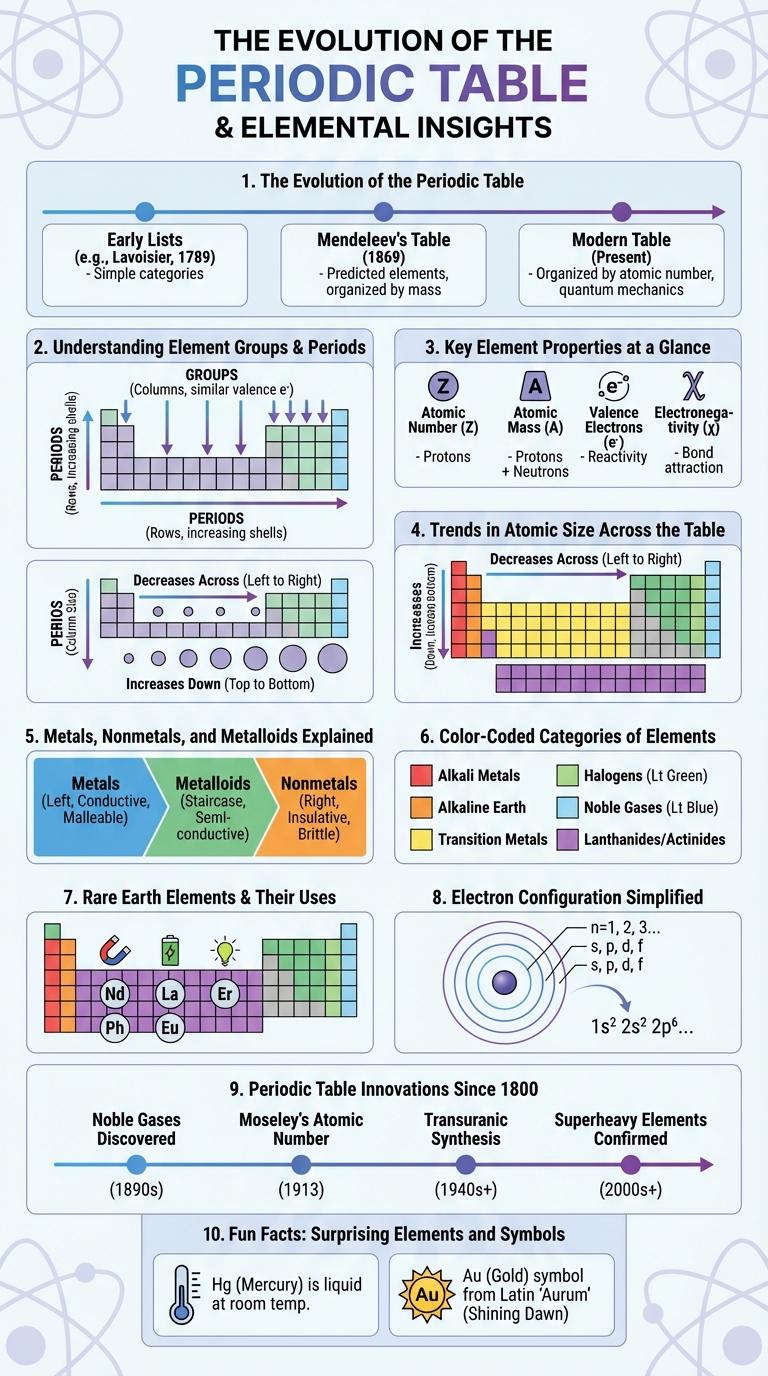
The Evolution of the Periodic Table
The periodic table has evolved significantly since Dmitri Mendeleev first introduced it in 1869. Originally arranged by atomic mass, the table organized elements based on their chemical properties and predicted the discovery of missing elements.
Modern periodic tables are arranged by atomic number, reflecting a deeper understanding of atomic structure. This evolution incorporates new elements and reflects trends in electron configurations and periodicity.
Understanding Element Groups & Periods
The periodic table organizes elements into groups (columns) and periods (rows) based on their atomic structure and properties. Groups contain elements with similar chemical behaviors due to their valence electron configurations. Periods reflect elements with increasing atomic numbers and changing properties across each row.
Key Element Properties at a Glance
| Element Property | Key Details |
|---|---|
| Atomic Number | Represents the number of protons in an atom's nucleus, defining the element's identity. |
| Atomic Mass | Average mass of atoms, accounting for isotopes, expressed in atomic mass units (amu). |
| Electronegativity | Measure of an element's ability to attract electrons in a chemical bond, influences reactivity. |
| State at Room Temperature | Elements exist as solids, liquids, or gases at 25degC, impacting their applications and handling. |
| Group and Period | Group denotes vertical column with shared properties; Period indicates horizontal row with increasing atomic number. |
Trends in Atomic Size Across the Table
The periodic table reveals clear trends in atomic size when moving across periods and down groups. Understanding these trends helps explain the behavior and properties of elements.
- Atomic size decreases across a period - Atoms become smaller from left to right due to increased nuclear charge pulling electrons closer.
- Atomic size increases down a group - Additional electron shells cause atoms to grow larger despite nuclear attraction.
- Transition metals show slight size variation - d-orbital electron filling causes atomic radius to change less significantly across the block.
Overall, atomic size depends on the balance between nuclear charge and electron shell number, influencing element reactivity and bonding.
Metals, Nonmetals, and Metalloids Explained
The periodic table organizes elements based on their chemical properties and atomic structure. Metals, nonmetals, and metalloids are three main categories that exhibit distinct physical and chemical characteristics.
Metals are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals generally lack metallic luster and are poor conductors. Metalloids have properties intermediate between metals and nonmetals, making them useful in semiconductors.
- Metals - Located mostly on the left and center of the periodic table, metals are characterized by high electrical conductivity and ductility.
- Nonmetals - Found on the right side of the periodic table, nonmetals are usually gases or brittle solids with high electronegativity and low melting points.
- Metalloids - Positioned along the dividing line between metals and nonmetals, metalloids exhibit mixed properties useful in electronic devices and catalysts.
Color-Coded Categories of Elements
The periodic table organizes elements into distinct color-coded categories, making it easier to identify their properties at a glance. Each color represents a specific group such as metals, nonmetals, and metalloids.
Metals are typically shown in shades of blue and silver, highlighting their conductivity and malleability. Nonmetals and metalloids use contrasting colors like green and yellow to emphasize differences in chemical behavior and physical traits.
Rare Earth Elements & Their Uses
Rare Earth Elements (REEs) are a group of 17 chemically similar elements crucial for modern technologies. They are found in the lanthanide series on the periodic table and are essential in manufacturing high-tech devices.
- Neodymium - Used to produce powerful magnets essential for electric vehicle motors and wind turbine generators.
- Lanthanum - Integral in camera lenses and battery technologies due to its optical and electrochemical properties.
- Europium - Vital for producing red and blue phosphors in display screens and LED lighting.
Electron Configuration Simplified
What is electron configuration in the periodic table? Electron configuration describes the distribution of electrons in an atom's orbitals. It helps predict chemical properties and reactivity of elements.
How are electrons arranged in atoms? Electrons fill orbitals in a specific order based on energy levels. This order follows the Aufbau principle, filling lower energy orbitals first for stability.
Why is electron configuration important? It explains how elements bond and interact in chemical reactions. Understanding these patterns simplifies the study of the periodic table's structure and element behavior.
How can electron configuration be simplified? Using notation like noble gas shorthand shortens complex configurations. This method represents inner electrons with a noble gas core and lists only valence electrons.
| Electron Shell | Maximum Electrons |
|---|---|
| K (1st shell) | 2 |
| L (2nd shell) | 8 |
| M (3rd shell) | 18 |
| N (4th shell) | 32 |
Periodic Table Innovations Since 1800
The periodic table has undergone significant innovations since 1800, transforming from a simple arrangement of elements to a comprehensive scientific tool. These advancements reflect the growing understanding of atomic structure and chemical properties.
In 1869, Dmitri Mendeleev introduced the first widely recognized periodic table, organizing elements by atomic weight and predicting undiscovered elements. The discovery of atomic number by Henry Moseley in 1913 refined the table's accuracy, arranging elements by increasing atomic number instead of weight. Modern periodic tables incorporate electron configurations and categorize elements into s, p, d, and f blocks, enhancing predictive capabilities in chemistry.
